Hyaluronic Acid Does Not Hold 1000× Its Weight in Water: A Non-Crosslinked Gel Study
ABSTRACT
Background: Hyaluronic acid (HA) is a glycosaminoglycan with strong hydrophilicity, widely marketed with the claim that it “holds 1000× its weight in water.” This claim lacks support in peer-reviewed literature and typically conflates crosslinked fillers, mixed solvents, and marketing copy.
Objective: To determine the water-only gel point and saturation point of non-crosslinked HA at low and high molecular weights (LMW, HMW).
Methods: HA (LMW 0.8–1.0 MDa; HMW 1.0–1.5 MDa) was hydrated in deionized water (1–6% w/w). Gel flow was quantified at 30 s using a GLTL Standard Consistometer (ASTM F1080). Gel and saturation points were inferred from flow-distance inflection behavior.
Results: HMW HA saturated at ~6% (≈30× water:HA, w/w). LMW HA saturated at ~5% (≈20× water:HA, w/w). Below ~1% HA, stable gels did not form under these conditions.
Conclusion: In water and without crosslinking, HA binds on the order of tens—not thousands—of times its weight. These findings align with polymer physics expectations for non-crosslinked GAGs in aqueous media and contradict the “1000×” cosmetic claim.
INTRODUCTION
Hyaluronic acid (HA) is a negatively charged polysaccharide (glycosaminoglycan, GAG) abundant in the extracellular matrix (1–3). Its hydrophilicity and gel-forming behavior underpin clinical use in injectable fillers (4–5) and widespread cosmetic marketing. Despite pervasive claims that HA “holds 1000× its weight in water,” the primary literature typically involves crosslinked HA, mixed solvents, or composite hydrogels (6–9), limiting inference about intrinsic water-binding of non-crosslinked HA.
This study isolates the question: in deionized water, how much water can non-crosslinked HA incorporate at saturation, and how do molecular-weight differences affect gel formation? Using consistometry tailored to non-Newtonian systems (10), we identify gel and saturation points and estimate practical water-to-polymer ratios.
MATERIALS AND METHODS
Hyaluronic Acid
Non-crosslinked HA powders were used: HMW (1.0–1.5 MDa; CAS 9067-32-7) and LMW (0.8–1.0 MDa; CAS 9067-32-7).
Gel Preparation
Deionized water (RT) served as the sole solvent. For each HA type (HMW, LMW), gels at 1–6% (w/w) were prepared by adding 90 g DI H2O to pre-weighed HA, stirring until visually dissolved, sealing, and storing 24 h at 4 °C to equilibrate.
Gel Point and Saturation Point
Flow distance at 30 s was measured on a GLTL Standard Consistometer (ASTM F1080 / Mil-Spec R-81294D). Reduced flow distance corresponds to higher apparent consistency. Gel point was defined as the first pronounced inflection in flow distance versus concentration; saturation as the subsequent plateau/near-zero flow region.
Consistency Change Calculation
For consecutive concentrations ci, ci+1 with readings Ri, Ri+1 (cm), percent change was:
%Δ consistency = abs(Ri+1 − Ri) / Ri × 100
RESULTS
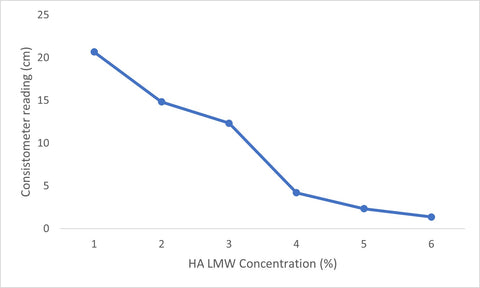
As HA concentration increased from 1→6% (w/w), consistometer flow distance decreased for both LMW and HMW, indicating thicker, more cohesive gels. LMW exhibited its first large inflection between 3→4%, while HMW exhibited a large inflection between 1→2%.
| Table 1. Consistometer readings (LMW HA) | |
|---|---|
| HA (LMW) concentration (%) | Flow distance at 30 s (cm) |
| 1 | 20.7 |
| 2 | 14.8 |
| 3 | 12.3 |
| 4 | 4.20 |
| 5 | 2.33 |
| 6 | 1.37 |
| Table 2. Consistometer readings (HMW HA) | |
|---|---|
| HA (HMW) concentration (%) | Flow distance at 30 s (cm) |
| 1 | 17.0 |
| 2 | 7.43 |
| 3 | 4.00 |
| 4 | 2.30 |
| 5 | 1.30 |
| 6 | 0.63 |

Estimated Water-to-Polymer at Saturation
- LMW: Saturation ~5% HA → ≈20:1 water:HA (w/w)
- HMW: Saturation ~6% HA → ≈30:1 water:HA (w/w)
Note: Below ~1% HA, samples behaved semi-liquid and were not reliably captured by consistometry under the 30 s readout.
DISCUSSION
Under water-only conditions and without crosslinking, HA’s saturation occurs at tens of times its weight—not at 1000×. Molecular weight modulated gelation: HMW achieved higher apparent consistency at matched concentrations and reached a higher water:polymer ratio at saturation than LMW. These results are congruent with prior observations that HA solutions are non-Newtonian and concentration-dependent (10) and that crosslinking substantially alters gel networks (6–8).
The consistometer provided a pragmatic, reproducible way to detect inflection behavior (gel point) and near-plateau (saturation) in a non-Newtonian system. While not a full rheometric sweep, the approach distinguished practical gel formation thresholds in a way that maps to real-world formulation limits.
Limitations
- Concentration range constrained to 1–6% for instrument practicality; below ~1% no stable gel formed, above ~6% movement was near-zero at 30 s.
- Flow distance is an indirect proxy; future work should include oscillatory rheology (G’, G”) and water activity/osmometry for corroboration.
- Only DI water was assessed; buffers, electrolytes, and temperature shifts may change network behavior.
Implications
Consumer-facing statements that HA “holds 1000× its weight in water” are incompatible with measurements on non-crosslinked HA in water. In topical skincare, reliance on HA for bulk water retention should be tempered with barrier-supportive strategies (lipid-balanced hydration, anti-inflammatory serums) as outlined in The OUMERE Routine. For a plain-language overview of this myth and why it persists, see: OUMERE Lab Article: HA ≠ 1000×.
Data Availability
Ouriel, Wendy (2022), “Hyaluronic Acid Data Set”, Mendeley Data, V1, doi: 10.17632/dt4p2c53xc.1
References
- Lee, D.H. et al. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci 83(3), 174–181 (2016).
- Juncan, A.M. et al. Advantages of hyaluronic acid in cosmeceuticals. Molecules 26(15), 4429 (2021).
- Alberts, B. et al. Essential Cell Biology, 3rd ed., Garland (2010), 698–699.
- Ilyin, S.O. et al. Rheology of HA injection implants. Rheol Acta 55(3), 223–233 (2016).
- Tezel, A. The science of HA dermal fillers. J Cosmet Laser Ther 10(1), 35–42 (2008).
- Xuejun, X. et al. Hydrogel from low-MW HA. J Bioactive Compat Polym 19(1), 5–15 (2004).
- Andre, P. HA as a rejuvenation agent. Semin Cutan Med Surg 23(4), 218–222 (2004).
- Wende, F.J. Structural studies of hyaluronan hydrogels. Uppsala Univ. (2019).
- Young, R.J., Lovell, P.A. Introduction to Polymers, CRC (1991), 306.
- Pisárčik, M. et al. Non-Newtonian properties of HA solutions. Colloids Surf A 97(3), 197–202 (1995).


